Abstract
Introduction
Emicizumab, a bispecific humanized monoclonal antibody in development for the management of patients (pts) with hemophilia A (PwHA) with/without inhibitors, is administered subcutaneously and facilitates effective hemostasis by bridging FIXa and FX to restore the function of missing FVIIIa. In the multicenter, randomized, phase 3 HAVEN 1 study (NCT0262232) in PwHA with inhibitors aged ≥12 yr, emicizumab prophylaxis vs no prophylaxis significantly reduced annualized bleeding rate (ABR; treated bleeds) by 87% (P<0.001), with 62.9% vs 5.6% of pts, respectively, experiencing zero treated bleeds (Oldenburg et al. NEJM 2017; July 10: epub). In intra-individual comparisons in pts who had previously participated in a prospective, non-interventional study (NCT02476942), emicizumab prophylaxis vs prior episodic and prophylactic bypassing agent (BPA) treatment significantly (P<0.001) reduced ABR by 79% and 92%, respectively (Oldenburg et al. 2017). During the study, serious thrombotic and thrombotic microangiopathy (TMA) events were reported in 5 pts, and were associated with aPCC doses >100 U/kg/day given for ≥24 hr for treatment of breakthrough bleeds (BTBs) during emicizumab prophylaxis; no events were reported when emicizumab was given alone, or in conjunction with rFVIIa alone (Oldenburg et al. 2017). To mitigate further risk of such events, the sponsor implemented dosing guidance to study investigators for BPA use during emicizumab prophylaxis. We describe treatment of BTBs in HAVEN 1 prior to and following BPA dosing guidance during emicizumab prophylaxis.
Methods
At the onset of HAVEN 1, pts were instructed to treat BTBs with BPAs according to standard of care, based on the emicizumab safety profile seen in the Phase 1/2 study. With the emerging TMA and thrombotic events, guidance provided on BPA use during emicizumab prophylaxis advised caution with using rFVIIa; it was recommended that BTBs preferably be treated with the lowest rFVIIa dose expected to achieve hemostasis, using ≤90 µg/kg/day as the initial dose. Pts/investigators were advised to avoid the use of aPCC. If aPCC was the only available BPA, use of the lowest dose expected to achieve hemostasis was advised. Also, local and central laboratory assessments were recommended to monitor for the risk of TMA or thromboembolic (TE) events.
Results
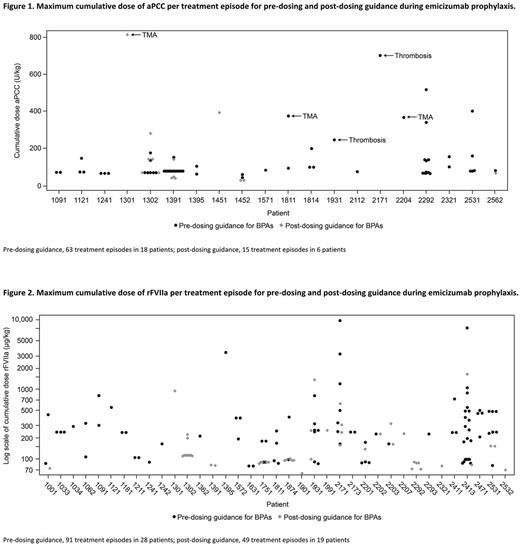
Median (range) emicizumab exposure was comparable for pre- (167.0 [20-334] wk) and post- (168.3 [28-186] wk) dosing guidance for BPA use periods. There were 63 aPCC treatment episodes in 18 pts pre-dosing guidance, and 15 aPCC treatment episodes in 6 pts post-dosing guidance (Figure 1). Prior to BPA dosing guidance, the majority of aPCC treatment episodes (44 [69.8%]) were ≤100 U/kg/day and consistent with 1 injection only; 43 (68.3%) were ≤24 hr. Of 19 (30.2%) treatment episodes >100 U/kg/day, 7 (11.1%) were ≥24 hr; 4 treatment episodes were associated with the reported TMA/thrombotic events (Figure 1). Post-dosing guidance, there were 6 (40.0%) treatment episodes <50 U/kg/day and 7 (46.7%) treatment episodes 50-100 U/kg/day, the majority for ≤24 hr. Of 2 (13.3%) treatment episodes that were >100 U/kg/day, 1 episode was ≥24 hr; this pt subsequently experienced a TMA event. The 5 treatment episodes associated with TMA/thrombotic events are shown in Figure 1.
There were 91 rFVIIa treatment episodes in 28 pts pre-dosing guidance, and 49 treatment episodes in 19 pts post-dosing guidance (Figure 2). Pre-dosing guidance, the majority (43 [87.7%]) of rFVIIa treatment episodes were ≤270 µg/kg/day for ≤24 hr. Pre-dosing guidance, 23 (25.3%) treatment episodes were >270 µg/kg/day. Post-dosing guidance, 37 (75.5%) treatment episodes were ≤180 µg/kg/day, with only 1 >24 hr in duration. Laboratory data from plasma samples collected subsequent to BPA dosing will be presented.
Conclusion
Dosing guidance implemented for BPA treatment during emicizumab prophylaxis with the emerging events of TMA/thrombosis in the HAVEN 1 study led to a decrease in the number of pts who used aPCC, with most switching to rFVIIa. Following dosing guidance, lower doses of aPCC and rFVIIa were used to treat BTBs effectively in pts on emicizumab prophylaxis; further TMA/TE events were successfully mitigated in pts who adhered to the guidelines. aPCC doses lower than the maximum dose within a 24-hr interval included in the prescribing information are advised when administered during emicizumab prophylaxis.
Callaghan: Sancillio: Other: Site PI; Octapharma: Membership on an entity's Board of Directors or advisory committees; CSL Behring: Membership on an entity's Board of Directors or advisory committees; Pfizer Inc.: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Other: Site PI, Speakers Bureau; Global Blood Therapeutics: Other: Site PI; Roche; Shire: Speakers Bureau; Grifols: Membership on an entity's Board of Directors or advisory committees; Bayer HealthCare; Pfizer Inc.; Roche; Shire: Consultancy; Alnylam Pharmaceuticals, Inc: Other: Owns stock, stock options, or bonds ; Bayer: Membership on an entity's Board of Directors or advisory committees; Baxalta: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novo Nordisk: Speakers Bureau; Shire: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Biogen: Membership on an entity's Board of Directors or advisory committees. Negrier: Alnylam: Research Funding; Shire: Honoraria, Research Funding, Speakers Bureau; Bayer: Honoraria, Research Funding, Speakers Bureau; Biogen: Honoraria, Research Funding, Speakers Bureau; CSL Behring: Honoraria, Research Funding, Speakers Bureau; Octapharma: Honoraria, Research Funding, Speakers Bureau; Novo Nordisk: Honoraria, Research Funding, Speakers Bureau; LFB: Honoraria, Speakers Bureau; Pfizer: Honoraria, Research Funding, Speakers Bureau; Roche: Honoraria, Speakers Bureau. Young: CSL Behring: Honoraria; Novo Nordisk: Consultancy. Khoo: Baxalta/Shire: Honoraria; Biogen: Honoraria; Novo Nordisk: Honoraria. Mahlangu: Roche: Consultancy, Research Funding, Speakers Bureau; NovoNordisk: Consultancy, Research Funding, Speakers Bureau; CSL Behring: Consultancy, Research Funding, Speakers Bureau; Catalyst Biosciences: Consultancy, Research Funding; Biogen: Research Funding, Speakers Bureau; Biotest: Speakers Bureau; Bayer: Research Funding; Baxalta: Research Funding, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Alnylam: Consultancy, Research Funding, Speakers Bureau; Shire: Consultancy, Research Funding, Speakers Bureau; Sobi: Research Funding, Speakers Bureau. Windyga: Novo Nordisk: Honoraria, Research Funding; Octapharma: Honoraria, Research Funding; Roche: Honoraria, Research Funding; Sanofi: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Shire: Honoraria, Research Funding; CSL Behring: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Baxter Healthcare: Honoraria, Research Funding; Biogen Idec: Honoraria, Research Funding; Baxalta: Honoraria, Research Funding. Oldenburg: Biogen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Grifols: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Octapharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Investigator Clinical Studies and Research Funding; Biotest: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Honoraria, Investigator Clinical Studies and Research Funding, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Baxter: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Xu: Genentech: Employment. Catalani: F. Hoffmann-La Roche Ltd: Employment. Asikanius: F. Hoffmann-La Roche Ltd: Employment. Adamkewicz: Genentech Inc.: Employment. Shima: Biogen: Consultancy, Honoraria; CSL: Honoraria, Research Funding; Chugai: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxalta: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Honoraria, Research Funding; Novo: Honoraria, Research Funding; Bayer: Honoraria, Research Funding; Kaketsuken: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal